While the Council will have no financial implications unless otherwise specified, its Institutes would provide support and facilitation to conduct the research and development.
| Photo Credit: Getty Images/istockphoto
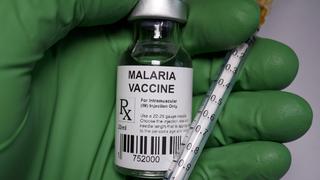
Indian Council of Medical Research (ICMR), Delhi, has invited Expression of Interest (EoI) from eligible organisations, companies and manufacturers for undertaking ‘Transfer of Technology’ for commercialisation of “a recombinant chimeric multi-stage malaria vaccine (AdFalciVax) against Plasmodium falciparum” useful in preventing Plasmodium falciparum infection in humans and minimizing its community transmission. The goal is to facilitate the commercialisation of this vaccine to prevent and minimize malaria transmission, and the EoI is open until August 17.
The Council noted that it would provide technical support through its team of experienced scientists in study planning, product development, development of study protocol, results/data analysis, outcome assessment, safety and efficacy assessment, product improvement, etc., if deemed fit upon mutual understanding between ICMR and the collaborative company.

Additionally, while the Council will have no financial implications unless otherwise specified, its Institutes would provide support and facilitation to conduct the research and development (R&D)/clinical study of new technology/product in India through its affiliates/institutes, in collaboration with the company/institutions. It would also provide technical support in development of technology/product and will also facilitate the validation, if required.
“ICMR-Regional Medical Research Centre, Bhubaneswar (ICMR-RMRCBB) is one of the four constituent institutes that has led the development of the technology and has technical-know-how of the process to produce this recombinant chimeric multi-stage malaria vaccine in Lactococcus lactis. The pre-clinical validation of this technology was conducted in collaboration with ICMR-National Institute of Malaria Research (ICMR-NIMR), another constituent institute of ICMR, and National Institute of Immunology (NII), New Delhi, an autonomous research institute of the Department of Biotechnology,” it added.
Published – July 19, 2025 08:29 pm IST













Leave a Reply