Imagine watching the brain not as a finished organ but as a city under construction, where every neuron is a worker changing jobs as the skyline rises.
A series of papers in Nature published on November 5 has captured exactly that. Led by researchers at the Allen Institute for Brain Science in the USA, together with partners across the US BRAIN Initiative, scientists have charted how the brain’s main cells — neurons and their supporting glia — form, migrate, and specialise across species from mouse to human.
Instead of treating the brain as a fixed catalogue of parts, the new maps portray it as a living continuum, a time-lapse of genetic patterns flickering on and off as cells mature, connect, and build networks.
For the first time these studies offer a unified view of brain development across time and species. Previous efforts were hard to compare because labs used different methods, sampled different stages or focused on separate regions. The BRAIN Initiative teams solved this by standardising protocols, building new sequencing and imaging tools, and creating shared computational pipelines to align data from mouse, marmoset, and human tissue. Together, they now provide a common reference for how neurons and glia emerge and assemble into circuits.
Hongkui Zeng, director of the Allen Institute, described it as ushering in a “new era of developmental neuroscience”, one that unifies data across space, time, and species. The six coordinated studies offer what she calls a “common reference” for how genes assemble the brain’s intricate circuitry, a guide likely to steer neuroscience for years to come.
Where old maps fell short
For decades, brain atlases treated neurons as if they came in fixed categories. The new datasets have overturned this view by showing that developing cells move through gradual transitions, with gene-activity patterns that change step by step rather than in sharp jumps.
In one of Dr. Zeng’s studies, her team found that as the mouse brain matured, young neurons passed through intermediate stages where they showed a mix of features from both earlier and forthcoming cell types.
“The boundaries are never clear-cut,” Dr. Zeng said.
Tomasz Nowakowski, an associate professor at the University of California, San Francisco, showed in his human lineage atlas that human brain development followed a similar path. By tracing the descendants of individual stem cells in cultured human foetal brain tissue, his team found that radial glia, the brain’s builder cells, first produced neurons that activate signals, then those that quiet them.
This gradual shift — which previous single-timepoint maps couldn’t see — confirmed that neurons don’t acquire their adult identity all at once.
Specifically, the two studies together showed that developing neurons don’t hold a single, stable identity. Their gene activity shifts gradually as they mature, passing through intermediate stages rather than jumping from one defined type to another.
Cells’ journeys
Dr. Nowakowski used viral barcoding to trace cell lineages in cultured human foetal brain tissue. The technique relies on harmless viruses that tag each stem cell with a unique genetic label, allowing researchers to follow all of its descendants.
His team then applied single-cell RNA sequencing to measure which genes were active in each developing neuron.
The team also used spatial profiling to place those gene readouts back into their exact locations within the tissue, almost like returning pins to a 3D map. Together, these methods created a time-resolved record showing how individual cells divided, differentiated and settled into their developmental paths.
Each of these steps began to show how one cell becomes many. Dr. Nowakowski said DNA analyses from postmortem human brains showed the same developmental shift his team observed in the culture, confirming that the pattern was not an artefact of the laboratory system.
Dr. Zeng’s computational atlas added a way to define developmental transitions more precisely. Her team used algorithms to detect when a neuron’s gene-activity profile had changed enough to qualify as a different cell stage, replacing earlier subjective judgments with quantitative criteria.
Finally, Yale University professor Rong Fan’s team added the missing dimension: place. In their spatial tri-omics atlas, the researchers measured three kinds of molecular information in thin, preserved slices of developing brain tissue: which genes were active, how accessible the surrounding DNA was, and which proteins each cell produced. Then they linked each measurement to the exact location of the cell within the tissue. This made it possible to see where different molecular patterns appeared and how neighbouring cells changed together over time.
Taken together, these approaches allowed the researchers to follow developing cells across both time and space, rather than capturing them at isolated moments.

Reading the new atlas
Together, these atlases have opened the door to understanding how billions of individual cell decisions shape the brain’s extraordinary diversity.
Cindy van Velthoven, an investigator at the Allen Institute, was part of a mouse study that tracked how inhibitory neurons, the cells that calm or balance brain activity, diversify and migrate as the forebrain forms. The team found inhibitory lineages that diversified at different times, some appeared later and were distributed across several regions, suggesting late-acting roles.
Building on his work, Dr. Nowakowski’s human atlas traced the complementary side of this circuitry: excitatory neurons, which increase neural activity. Read together, the two studies reveal how the brain’s opposing systems of excitation and inhibition take shape through continuous, overlapping pathways of gene expression.
Those same molecular networks also appear to have deep evolutionary roots, an idea explored by Alex Pollen, an associate professor at University of California, San Francisco.
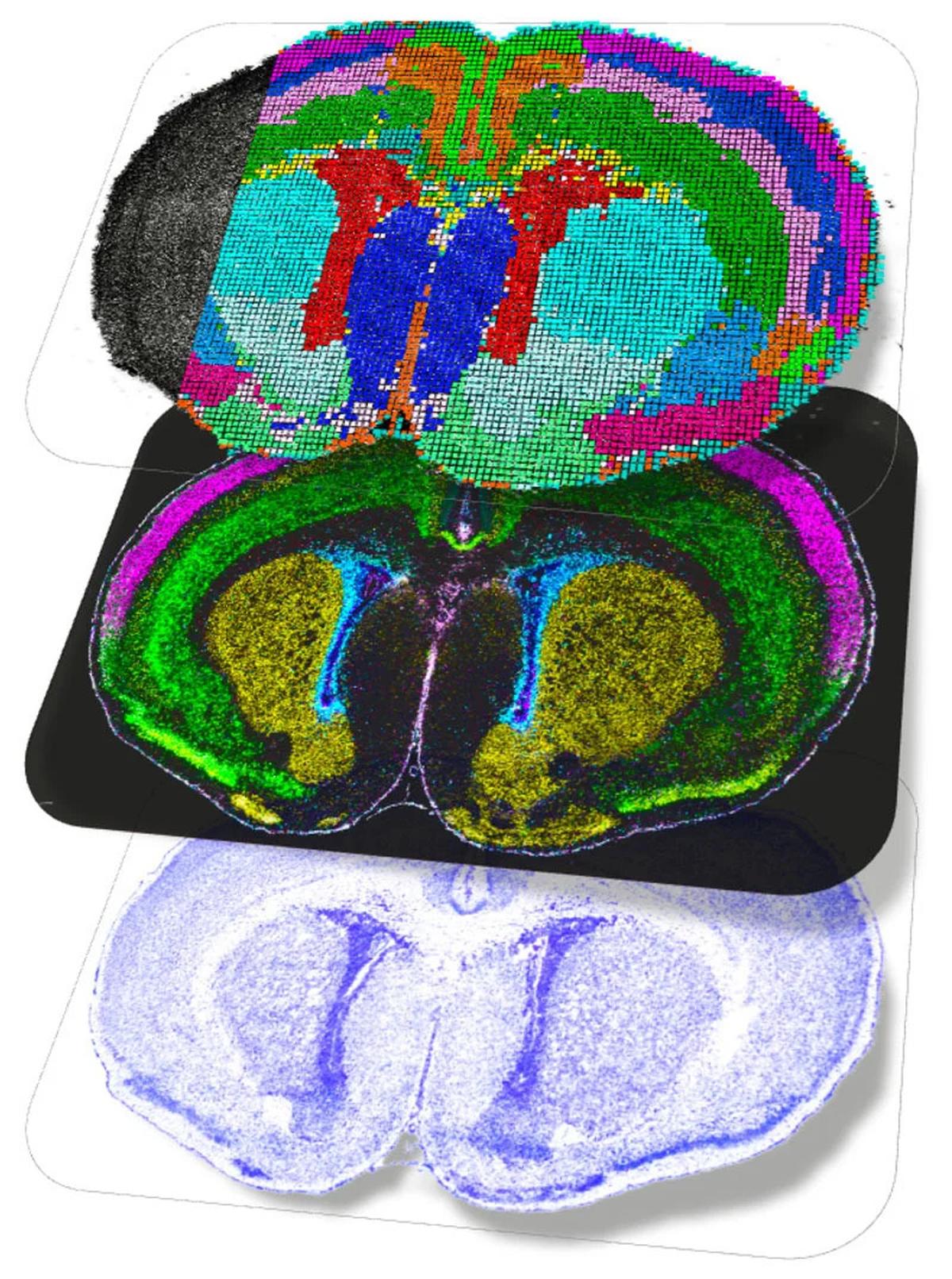
Postnatal mouse brain spatial multiomics maps highlighting cortical layer neuron maturation and oligodendrocyte differentiation and myelination.
| Photo Credit:
Di Zhang from Yale University; Zhang et al., Nature (CC BY)
In a cross-species analysis, Dr. Pollen and his colleagues compared gene activity across mammals and found that a neuron type once thought unique to primates, the TAC3 interneuron, which helps regulate emotion and hormonal signalling, is present across many mammalian lineages, though its abundance and molecular profile vary.
“The strongest evidence for shared ancestry came from looking broadly, from marsupials to primates,” Dr. Pollen said.
All these findings show that evolution tends to modify existing neuron types rather than create entirely new ones. In humans, similar developmental pathways are present but progress over a longer time, giving cells more time to diversify and form complex circuits.
Across species, the underlying pattern is consistent: developmental programmes are reused and adjusted, not replaced, to build the brain’s wiring.

Bringing it all together
With the individual atlases in place, the consortium took one final step. In the meta-atlas project led by Dr. Nowakowski, Dr. Zeng, and training programme mentor Aparna Bhaduri at the University of California, Los Angeles, the researchers aligned developmental data from mouse, marmoset, and human brains to create a shared reference that allows cell states to be compared across species.
Dr. Zeng acknowledged that “a lack of brain tissue, especially human samples from key stages of development, may be the biggest limitation for now.”
She said overcoming such gaps means constantly improving both the data and the tools used to analyse it: “We should not treat taxonomies rigidly, but continue to refine them as we gain new knowledge.”
For Dr. Bhaduri, the goal is to build a shared resource the entire field can use.
“Having this reference data is a fantastic opportunity for the field,” she said. “It will empower us to have common gene signatures, cell names, and analytical tools to move the field forward.”
Their project treats brain mapping as a collective, ongoing effort rather than as a finished product. As Dr. Zeng put it, the aim isn’t to finish the map but to ensure everyone is using the same coordinates.
From maps to medicine
For neuroscientists, these maps offer a clearer view of how early development sets the conditions for later brain function. They show when key genetic pathways switch on or off during gestation and how those shifts guide cells into specific roles.
The atlases highlight periods of development in which many genes associated with neurodevelopmental disorders are highly active, which could help researchers pinpoint when small disruptions are most likely to have long-term effects. Conditions such as autism or epilepsy are thought to involve changes in early developmental timing rather than damage that occurs later in life.
Dr. Nowakowski said the next step is to test whether the developmental switches his team observed also occur in other systems. Non-human primates, he noted, “may be the closest in-vivo model” while “organoids are another emerging model,” and he said he looks forward to seeing if the results align.
Beyond genetics and timing, the surrounding tissue environment also affects how cells mature. In the spatial atlas prepared by Fan et al., the team compared maps of gene and protein activity and found that cells in regions with stronger developmental signalling matured sooner while those in quieter regions developed more slowly. When they examined injured tissue, the brain activated patterns of gene activity similar to those seen during early development, suggesting shared mechanisms between growth and repair.
Researchers increasingly believe many neurological conditions arise when developmental events occur at the wrong time or in the wrong place, such as cells arriving too early, maturing too quickly or settling in an unusual location. The new atlases make these vulnerable periods easier to identify, pointing to specific stages in which small disruptions may have long-term consequences.
Work still ahead
Some neuron types appear only briefly or only switch on their defining genes in specific conditions, such as after recent activity or during particular behavioural states. Dr. Van Velthoven said such fleeting or condition-specific neurons “likely remain unseen” in today’s datasets.
Dr. Zeng agreed the journey is far from over: “Extending our work to the whole brain, including both the cortex, the brain’s outer layer, and the deeper subcortical regions that coordinate movement and emotion, would be the first step.”
“We need more timepoints and brain regions to construct a much more detailed framework for how the brain ultimately emerges,” Dr. Bhaduri said.
Together, these gaps define the next steps for the field: broader brain regions, more developmental stages, and denser sampling to capture cell types that current methods miss.
Anirban Mukhopadhyay is a geneticist by training and science communicator from New Delhi.













Leave a Reply